OVERVIEW
Bone healing, also known as bone healing, is a normal mechanism of the body
About 2 million cases fracture long is treated in USA each year. Of these, about 100,000 are non-healing. Failure to heal can cause a lot of problems not only for the patient but also for society at large. Patients without bone healing have significant disability and the associated costs of treatment are a burden to the patient and society.
Brinker has reported significant physical and mental disability related to tibial non-fusion. Although patients undergoing successful nonhealing therapy improve significantly, they often have reduced functional scores compared with the general population.
Antonova et al found that the average total cost of care for a case of tibia nonhealing was more than double the costs associated with a single case. tibia fracture which then occurs normal bone healing. In addition, the duration of drug use in patients with non-healing bone was twice as long as in those with normal bone healing (5.4 months versus 2.8 months).
Prosthetic joints, delayed bone healing are abnormal developments of a fracture with the ratio of 2 – 3% and 4 – 5% respectively, due to many causes: initial trauma factors, factors related to patient and by the physician.
Although orthopedic surgeons may be primarily responsible for the treatment of nonhealing bone, the coordinated involvement of multiple specialties is often necessary, including infectious disease physicians, surgeons, and other medical professionals. cosmetologist, endocrinologist, internist, physical and occupational therapist, psychiatrist or other mental health professional. The treatment of patients with nonhealing bone is often complex, but it is also highly rewarding because many of these patients have been significantly incapacitated over the long term.
Sir John Charnley's quote still holds true: "The best treatment for bone loss is prevention".
THE CONCEPT
According to Einhorn. CORR (1998), no bone healing is the process of bone healing stops, bone healing does not occur. Delayed healing is an ongoing process of bone healing, but not according to the desired time and bone healing is unlikely to be achieved.
Slow bone healing can easily lead to non-healing without further intervention. Therefore, when the diagnosis of slow bone healing is diagnosed, it is necessary to actively intervene to increase the possibility of bone healing.
The assessment of fracture healing time varies among surgeons. The healing time depends not only on the type of fracture, the location of the fracture, the characteristics of the injury and the quality of the soft tissue around the fracture, but also on the physiological capabilities of each patient.
The FDA (US Food and Drug Administration) (1986), defined a prosthesis as a broken bone that did not heal after 9 months of injury and did not show progress in healing after the series. X-ray through 3 consecutive months of follow-up.
Once a diagnosis of prosthetic joint is made, it implies that the broken bone will not heal without therapeutic intervention. The final condition of a non-healing fracture is the formation of a pseudoarthrosis (pseudarthrosis).
In fact, the word "false joint" is only for cases where the bone is not connected, has abnormal movement, is painless because there is a viscous pocket between the two ends of the broken bone covered with fibrous or cartilage tissue, which develops after a while. For a long time, there is no bone connection, also called synovial pseudarhrosis, and most people use the word non-union. However, due to habit, most people use the word pseudo-joint to imply that there is no bone healing.
BACKGROUND
The process of bone healing is a complex process involving many factors from the molecular and cellular level to the damaged area to the whole body. In terms of histology, the normal bone healing process takes place through four stages as follows:
The first stage is also known as the inflammatory phase
- Appears immediately after the fracture this phase lasts for 3 weeks with a peak on the 3rd to 5th day after the injury.
- The force of a fracture will simultaneously damage both the periosteal and bone marrow blood supply systems leading to caseation cells at the fracture site, these cells will release vascular wall activating factors causing increased vasodilation and vascular permeability. This process increases blood flow to the fracture site and peaks 2 weeks after the injury. Against the background of a blood clot formed from inflammatory cells, fibroblasts appear to produce collagen, which gradually replaces the clot with granular tissue.
The second stage is the bone-building phase: lasting from 1 to 4 months, including two stages as follows
- Formation of soft bone: takes place in the first 1-3 weeks:
- During this stage many neovascularizations are formed by the bone marrow stem cells. These stem cells enter the lesion and differentiate depending on local conditions: tissue oxygen concentration, tensile strength and local growth factors.
- Local tension activates fibroblast stem cells. In places with low oxygen levels and regular stretching, stem cells will create chondrocytes, then the cartilage cells will create a bridge between the two ends of the broken bone. reduce stretch and lead to bone healing.
- Osteoprogenitor cells will proliferate rapidly in an oxygen-rich environment and with little mechanical stress, these regions form the direct hardening of the bone.
- The soft bone is created by the transformation from granulosa tissue to temporary calcified tissue, including osteoblasts and chondrocytes and a system of collagen fibers. Osteoblasts and chondrocytes synthesize bone and cartilage-like intercellular substances. The mineralization of the soft can first occurs at the junction of the fracture ends, sequentially from one end of the fracture to the other until the two ends of the fracture are joined together. The can at this stage is very soft and fragile.
- Formation of hard bone: soft bone continues to grow, cartilage cells and collagen fiber system deposit calcium to create an environment for stem cells to enter and transform into osteoblasts, these cells transform. Cartilage has mineralized into rigid bony rafts arranged along the microtubules. The ossification forms rigid rafts to ensure a solid fracture connection.
The body repair phase can
The appropriate Havers bone is oriented to replace the hard bone (this process takes one to several years, returning the bone to its histological structure). Under the influence of mechanical forces, the bone structure here has a change in shape to suit the function of the bone. Repair is performed by bone modelizing units (BMUs) consisting of osteoclasts and osteoblasts and occurs in a repetitive sequence.
The period of recovery of the original bone shape
Lasts from one to many years. Bone shape recovers completely in children, but in adults it cannot be restored to its original shape.
Clinically and X-ray, corresponding to the stage of inflammation and granulation formation, manifested by swelling, heat, redness, pain, which gradually decreases after 7-10 days, X-ray of broken bones is still sharp. there is no change. Corresponding to the soft cannula formation stage, there is no clinical abnormality at the fracture site, the X-ray of the fracture ends is no longer sharp,
The fibula begins to appear (grade I bone can), gradually the bone can develop to form a bridge connecting the two broken ends, however, the fracture is still clear (grade II bone can). Corresponding to the stage of hard bone, can clearly be palpated clinically, no longer abnormal movements, no more pain at the fracture site, X-ray shows a large, strong bone mass connecting the two broken ends, no more gap gap (grade III bone can).
Rehabilitation process related to fracture treatment
The “diamond concept”, coined by Giannoudis et al in 2007, to describe what is needed to achieve fracture healing, consists of three biological factors (triangular concept): osteoblasts, bone scaffolds, growth factors and four mechanical stabilizing factors. If one of these factors changes, it will threaten the ability to heal the bone.
Fractures are treated conservatively and those treated with intramedullary nails, bridging splints, and various external fixators rely on secondary healing. Relative stability is provided by these devices when trying to achieve secondary healing. These fractures heal with scar tissue formation and progress through stages:
(1) inflammatory phase, (2) soft callus phase, (3) hard callus stage, and (4) regenerative phase. The inter-piece movement is usually in the range of 0.2 – 1 mm. Fractures are firmly fixed with screw splints based on direct bone healing.
Absolute stability is required; the inter-piece movement is less than 0.15 mm, the tension is less than 2% and the fracture gap is less than 0.1 mm. Healing is similar to the regenerative phase of secondary bone healing, with osteoclasts converting woven bone into lamellar bone. In many cases of splint fixation, the fracture gap is greater than 0.1 mm and primary healing does not occur. In this situation, a third type of healing occurs, void healing. With gap healing, the tension is still less than 2%; however, gaps up to 1 mm are acceptable.
CAUSES AND RISK FACTORS
Initial trauma factor
Has a lot of influence on the formation of slow healing and prosthetic joints such as: many displaced fractures, broken open, bone loss, fracture due to high energy trauma, infection. The rate of delayed bone healing increased with the severity of the open fracture. A factor that often determines the rate of bone healing is the integrity of the bone feeding vessels, especially for the tibial body; The destruction of the bone feeding artery when the fracture is displaced or the destruction of the periosteum in the second and third degree open fractures also increases the rate of prosthetic joints and slows bone healing. In a study of 842 cases of long bone prostheses, Boyd, Lipinski and Wiley found that prostheses were common in open fractures, infections, multi-segment fractures and loss of medial feeding vessels, complex fractures due to high-energy trauma, Adjustment is not good, fixation is not guaranteed, motionless not enough time, open the fracture or stretch the fracture. Through 814 cases of prosthetic joints, d'Aubigne also emphasized infectious factors as the cause of prosthetic joints. LaVelle studied over 300 cases of tibial fractures treated with intramedullary nails, found only 2% prosthetic joints and often occurred in patients with fractures of the ankle, tibial plateau or ipsilateral acetabulum, which did not allow early impingement.
The reason for the doctor's side
Often due to poor alignment of the fracture, the immobilization of the fracture is not stable, during surgery, the hematoma is lost, causing further damage to the blood vessels feeding the bone or dissecting and destroying much of the periosteum. Another common cause is that doctors follow up and treat after trauma not as indicated, such as removing the powder too soon or giving the patient a bed too late. Heppenstall, Brighton, Esterhai and Muller studied on 185 cases of tibial prosthesis and found that 92.4% had a late onset time of more than 6 weeks.
Patient-related factors
Tình trạng suy dinh dưỡng, chuyển hóa kém của bệnh nhân. Hút thuốc lá cùng với lượng nicotin hấp thụ gây ra tác dụng tiêu cực đối với quá trình liền xương được thấy cả trên lâm sàng và thực nghiệm. Một số bệnh lý toàn thân có thể ảnh hưởng như giảm miễn dịch, lao phổi, đái tháo đường. Việc không tuân thủ đúng chỉ định điều trị cũng góp phần tăng tỉ lệ khớp giả, chậm liền xương như bệnh nhân tự tháo bỏ bột, đi lại sớm khi can chưa đủ vững.
CLASSIFICATION OF JOINTS
In terms of academic organization
A tight prosthesis, also known as a fibrous prosthesis, is a type of prosthetic joint in which the two ends of the broken bone are connected by a fibrous organization: the gap between the two broken ends is usually small, so there is little mobility.
True prosthetic joint: two broken bones form a joint that closely resembles a real joint. The prosthetic joint is surrounded by a fibrous capsule resembling a joint capsule, which contains a sticky substance resembling synovial fluid. This type of prosthetic joint has a wide range of motion.
Loss of bone: The two ends of the broken bone have a large gap of 2 cm or more because the broken bone is thrown away, or removed during surgery.
In terms of bacteriology
Sterile prosthetic joint. Infected prosthetic joint.
In terms of software at the prosthetic drive
The prosthetic joint has normal software.
The prosthetic joint is covered with bad software, ulcer scars, adhesions, etc.
In terms of displacement
The prosthetic joint does not move. Displaced prosthetic joint.
X-ray classification
Judet and later Muller divided the prosthesis in general into two main categories: hypertrophic and atrophied according to the degree of growth of the head bone.
- Hypertrophic or hypervascular prostheses are biologically reactive prostheses. The Strontium 85 introduction study in this type of pseudohypertrophic joint showed rich vascularity at the fracture tip. This hypertrophic joint is further divided into three subtypes (Figure 4.2):
- Elephant foot non-union
- Horse foot non-union
- Oligotrophic non-union joint.
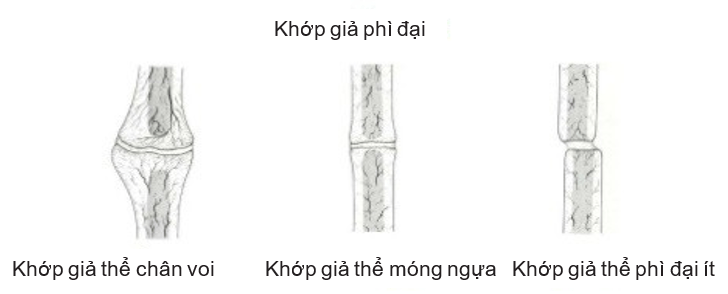
Figure 4.1. Hypertrophic prosthetic joint (Source: Ravi Varma, Ravi Kiran. Non-union, 2017)
- Atrophy or avascular prosthetic joint: a prosthetic joint that is inert or bioreactive. Introduction Strontium 85 in this type of atrophy prosthetic joint shows poor vascularization at the fracture tip. This atrophic pseudo-joint is further divided into four subtypes (Figure 4.2):
- Torsion wedge non-union 3rd piece
- Communized non-union joint
- Defect non-union joint
- Atrophic non-union joints.
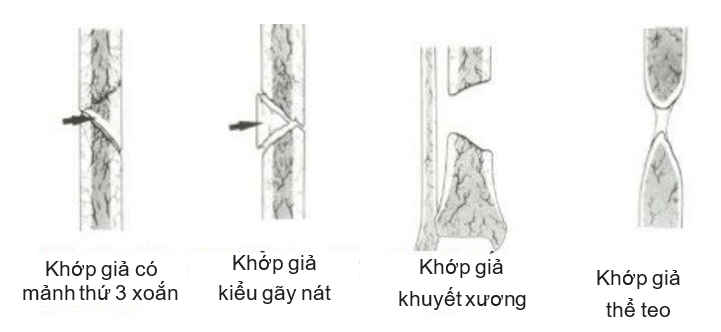
Figure 4.2. Atrophied prosthetic joint (Source: Ravi Varma, Ravi Kiran. Non-union, 2017)
CLINICAL AND PHOTOGRAPHY
clinical
For fractures without internal bony fusion:
- Usually there is more or less pain in the joint socket
- Unusual movements
- Decreased limb muscle.
For fractures that have been combined with bones, there may be pain and more or less reduced limb function.
X-ray
There is a gap between the two ends of the broken bone. The canal is occluded, the broken end has a thick fibrous bone border (selerose). Depending on the type of prosthetic joint, this bone border is thick or thin. The broken ends may be atrophied, mortise-shaped, or as wide as an elephant's foot.
Slow bone healing, the X-ray image simply shows the gap, but the ends of the bones have not been fibrous.
DIAGNOSE
Slow bone healing
By the time of bone healing, the following signs are still present:
- Swelling at fracture site
- Sharp pain when pressed or when the broken limb is active
- Abnormal movements
- X-ray: there is a light gap between the two broken ends.
Prosthetic joint
More than twice the healing time:
- There are signs of loss of power
- Completely painless when pressing or moving the broken limb
- Abnormal movements are still there
- X-ray: The two broken ends are still spaced, the broken ends are fibrotic.
TREATMENT OF JOINTS
General principles
Create contact of two broken ends, strengthen immobilization to help restore normal bone healing.
Detect and eliminate factors that interfere with bone healing.
Create conditions for blood vessels to grow and penetrate the fracture site, providing more bone-building materials.
Improve the general condition of patients against malnutrition disorders.
Therapeutic measures
Slow bone healing
Enhance immobilization, fix the fracture with a longer time.
Bone grafting by Phemister method: placing spongy or hard bone grafts under the fascia to bridge the two broken ends.
Beck-style bone drilling: Use a Kirschner nail to drill multiple holes through the broken ends.
Prosthetic joint
Tight prosthesis: bone grafting by Phemister, Matti method, or stretch and compressive fusion with external fixed frame.
Synthetic fibrous joint: refresh the broken ends (remove the fibrous parts, drill the canal) and then metallize bone graft or peel the bone according to Judet's method. R (decortication).
Với các khớp giả mất đoạn xương dài có thể dùng phương pháp kết xương hai ổ (một ổ căng dãn và một ổ nén ép) hoặc ghép xương có cuống mạch nuôi bằng kĩ thuật vi phẫu, hoặc kết ghép xương theo các kĩ thuật kinh điển.
With infected pseudoarthrosis: after removing the necrotic inflammatory tissue and dead bone fragments in the prosthetic joint, it is possible to carry out bifocal bone grafting or bone grafting with vascular pedicle.
Bone grafting methods and biology
- Autologous bone grafting: is considered the gold standard in the treatment of prosthetic joints, providing a high healing rate of 80-90%. The iliac crest graft contains all the necessary elements such as 15% living osteoblasts and other mesenchymal cell lines, on the other hand, provides the bone-building materials immediately, so it is able to create new bone right after transplantation. . However, this method has limitations because of the number and inconveniences in the area of bone grafting.
- Bone marrow transplantation and autologous bone marrow stem cells: contain many stem cells capable of transforming into osteoblasts. This ability has been applied by many authors to enhance the osteogenic properties of bone grafts by mixing grafted bone with bone marrow obtained during surgery. Hernigou et al. (2005) reported 60 cases of tibial stem prostheses receiving autologous bone marrow transplantation using centrifuge to increase the number and concentration of transplanted stem cells, the healing rate reached 95%, showed that this is a minimally invasive, simple and effective method.
- Biostimulation methods: In general, still in the process of research and testing, some methods such as platelets - autologous growth factors, growth and differentiation factors 5 (growth and differentiation factor-5), low frequency ultrasound.
- Allogeneic bone grafting: the method is increasingly applied due to many advantages such as: large size grafts, many shapes and different locations, no complications in the extraction area, providing materials for the process. bone regeneration. The disadvantage is that it only provides materials but does not participate in the bone regeneration process, depends on technology and equipment, high risk of infection due to transplant rejection.
- Graft of bone-forming derivatives: ceramic and hydroxyapatite, collagen, coral preparations, beta-tricalcium phosphate. Only an auxiliary effect provides materials for bone formation, so it cannot be used alone and is still under research.
Treatment of infected prosthetic joints
Considerations when treating infectious prostheses. Classically, two completely different treatment approaches have been most commonly used for this difficult problem. The first is the standard, or classic, method, used for decades. The second is the proactive approach. Part or all of these methods may be performed, depending on the given patient and the surgeon's judgment. The two are described separately, but surgeons often apply each method only partially on a patient. The Ilizarov method is another method for the treatment of infected prosthetic joints that have similarities with the standard methods and the method of operation. The condition involving the bone (bone marrow, superficial, localized, and diffuse) and the patient's body's ability to help the surgeon determine the healing ability of the infected bone. The gold standard for diagnosing infection is multiple direct implantation at the fracture site (not skin or fistula). However, a recent report has questioned the sensitivity of the cultures in diagnosing infection in the treatment of nonhealing bone.
Standard treatment:
- The goal of this approach is to transform an infected and fistula prosthesis into a leak-free prosthesis within months and promote healing of the prosthesis by bone grafting. This treatment usually requires a long time and multiple surgeries. Excision is performed with the removal of all foreign, infected, or damaged material to create a vascular bed. At this point, the provision of some stabilizing factor is considered with relevance provided by the plastic surgery team. Infection can be more easily controlled using healthy, vascular soft tissue to cover the fracture, especially in cases of tibial prosthesis. Initial external fixation may be most appropriate. Antibiotics are administered parenterally and are culture-based during surgery. The bone graft is deferred until the soft tissues have completely healed and become stable. In some patients, the fracture heals and bone grafting becomes unnecessary.
- When the infection is active mainly in the soft tissues or around the tissues, the risk of reactivating it surgically is much less than if it already involves the cortex and the canal. As the infection persists and destroys, all surrounding structures are said to have become deeply infiltrated and potentially become a dormant infection. The bacteria can lie dormant for years, only to become active again after surgery or some other trauma. This hazard is inherent in the handling of infectious prostheses and must be accepted. The use of antibiotics before and after surgery has reduced risk because they can often control infection within the confines of a vascular area, but they cannot disinfect an avascular area that they cannot penetrate. import. Reconstructive surgeries should usually be delayed until at least 6 months after all signs of infection have disappeared.
Active treatment:
- The goal of an active approach is to get early bone healing and shorten the healing time and preserve movement in adjacent joints. Judet and Patel and Weber and Cech described this approach, and much of the following is excerpted from their report.
- The first step is to restore bone continuity. This takes absolute precedence over treating the infection. The prosthesis is exposed through old scars and fistulas. The ends of the fractures are peeled to subperiosteal, forming many small bone fragments; Any pieces that are split will be discarded. Next, all infected and damaged soft tissue and bone will be removed. The pieces are then aligned and stabilized, usually with an external fixation device. Compression is applied on the prosthetic joint if possible. Autologous spongy bone can be implanted. Internal fixation with a splint is used only when the fistula is occluded and when the approach is far from the area of the old fistula or when immobilization cannot be achieved by other methods and the infection is mild. Once the fracture has been secured with a splint or intramedullary nail, fixation is undisturbed and the operation is performed as described except that decapitation is omitted when an intramedullary nail is used. The wound was then closed and culture-based systemic antibiotics were administered intraoperatively.
- If necessary for bone healing, bone removal with or without the addition of autologous bone grafts is performed. After the prosthesis has healed, any residual damage is removed and a semi-thick skin graft is applied to any remaining defects in the skin. Positive outcomes have been reported with or without autologous bone graft, with success rates ranging from 83% to 98%.
Polymethyl methacrylate antibiotic bed:
- Polymethyl methacrylate (PMMA) sheets impregnated with antibiotics can be used to treat infected prosthetic joints. Thonse and Conway found that Palacos cement (Zimmer, Warsaw, IN) has a higher permeability than Simplex (Stryker, Mahwah, NJ). Heat-stable antibiotics, such as tobramycin and gentamicin, can be mixed with PMMA and used topically to achieve 200 times the antibiotic concentration when given intravenously. One study has shown that the use of antibiotic-impregnated PMMA sheets in combination with antimicrobials in the management of sepsis prosthetic joints is more effective in treatment than parenteral antibiotics. The placement of the PMMA spacer is another option that is likely to provide some stability in a bone defect situation. The body's response to PMMA sheets or pads leaves a bioactive film, the Masquelet membrane. The use of grafted cancellous bone to deliver antibiotics to uninfected sites has been described in a small number of patients with favorable results; however, the optimal ratio of antibiotics to cancellous bone is not known.
CONCLUDE
The healing process is a complex process involving many factors. A thorough understanding of the healing process and grafting is extremely important in clinical practice to help the treating physician make appropriate adjustments to provide optimal bone healing results for the patient.
The choice of treatment method depends on many factors such as disease status, actual conditions, experience and equipment in each place. Although there are many other methods, autologous bone grafting with iliac crest graft is still the gold standard in the treatment of delayed healing and prosthetic joints.
In recent years, research on stem cells has received a lot of attention and promising results in biomedical research have been achieved thanks to their ability to use these cells to generate replacement cells for damaged cells. damage. The method of autologous bone marrow transplantation using centrifuges to increase the number and concentration of transplanted stem cells has a healing rate of 95%, showing that this is a less invasive, simple and effective method. .

bài viết rất hay ạ
Dạ. bài viết rất hay
Cảm ơn thầy nhiều ạ
Bài giảng hay lắm ạ
Dạ. bài viết rất hay
Bài viết rất hay ạ